PUBLISHED THE SIXTEENTH AMENDMENT RELATING TO REGULATION (EU) 10/2011
PUBLISHED THE SIXTEENTH AMENDMENT RELATING TO REGULATION (EU) 10/2011
The Regulation (EU) 2023/1442 of the Commission of July 11th 2023, wich amends Annex I of Regulation ( EU) n.10/2011
concerning food contact plastic material and objects , as regards changes to the authorizations of substances and the
addition of news substances, was issued by the European Union on July 12th 2023.
The amendment issued by the European Union on July 12th 2023, provides for significant changes, such as:
– Plastic materials and objects manufactured with salicylic acid (MCA substances N.121) or manufactured with
untreated wood flour and fibres (N. 96), can continue to be placed on the market starting from 1 February 2025,
provided the meet certain conditions also relating to Regulation (EC) n. 1935/2004 and only under validation by the
European Authority. These substances have been removed from Annex I of Regulation (EU) N.10/2011.
-Five new entries have been added to Annex I as FCM substances n.178 and 1080,1081,1082,1083.
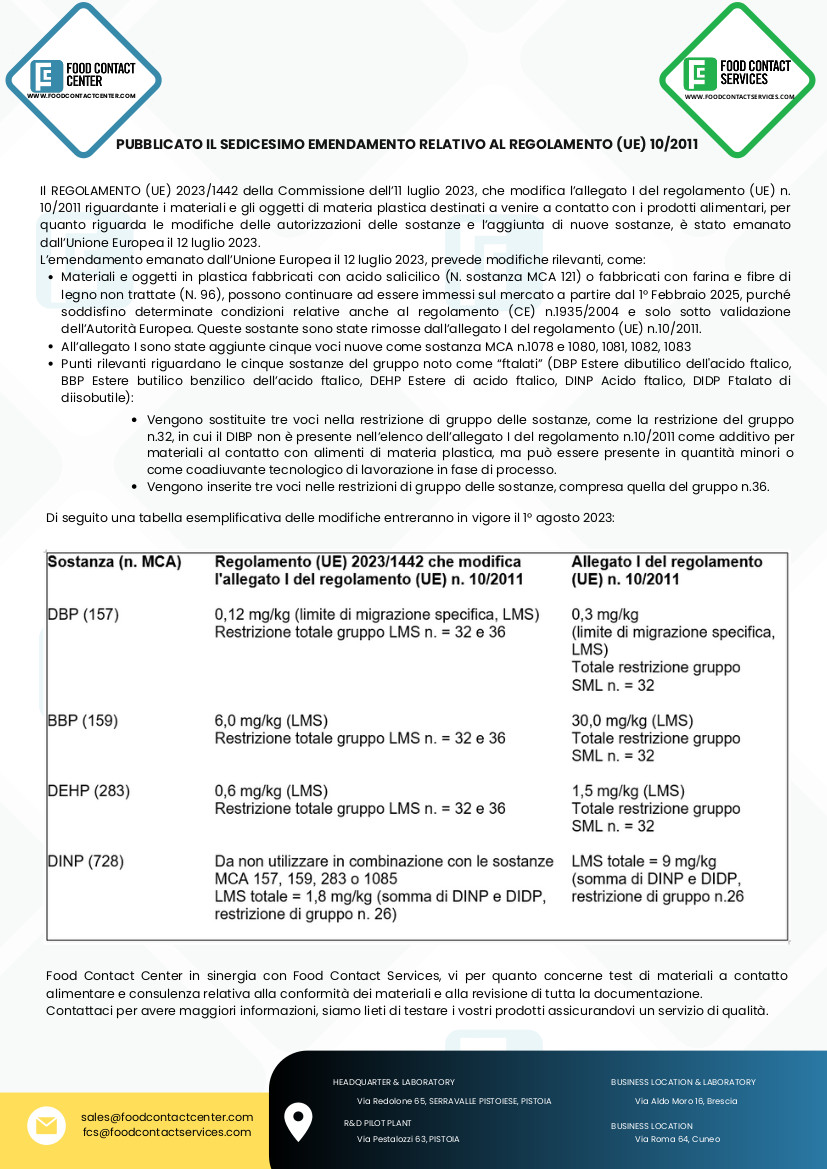
– Relevant points concern the five substances of group Known as “phthalates” (DBP Phthalic acid dibutyl ester, BBP
Phthalic acid butyl benzyl ester, DEHP Phthalic acid ester, DINP Phthalic acid, DIDP Diisobutyl phthalate):
- Three entries in the group restriction of substances are replaced, such as restriction group n.32, where DIBP is not listed in Annex I of Regulation n.10/2011 as an additive for food contact materials of plastic material, but it can be present in smaller quantities or as a technological processing aid in phase process.
- Three entries are made in the substance group restrictions, including that of group n.36.
